Botany online 1996-2004. No further update, only historical document of botanical science!
Field Work With Plants
Phil Gibson, Department of Biology, Agnes Scott College, Decatur, GA, 30030. http://ecademy.agnesscott.edu/~pgibson/ 404-471-6267 |
Field experiences provide students with the opportunity to get out of the lab (obviously) and gain a perspective on botany that is not obtainable from lab based exercises. Although thorough planning is necessary for success, field-based lab exercises are generally easy to develop and can be designed to achieve a variety of goals.
Why field experience?
Examples of field exercises
Resources For Field Experiences With Plants
Texts
Avery, T. E. & H. E. Burkhart. 1983. Forest measurements. 3rd ed. McGraw-Hill. New York, NY. 331 p.
Barbour, M.G., J.H. Burk, and W.D. Pitts. 1987. Terrestrial Plant Ecology. 2nd ed. Benjamin/Cummings Publishing Co., Menlo Park, California. {This text provides more detailed discussion and descriptions of most of the techniques and theories described in this lab exercise.}
Brower, J.E., J.H. Zar, and C.N. von Ende. 1998. Field and Laboratory Methods for General Ecology. 4th ed. WCB McGraw-Hill Publishers. Boston, MA.
Durrell, G. 1989. The Amateur Naturalist. Alfred A. Knopf. New York, NY. 320 p.
Web Sites
Biodiversity
International Biodiversity Observation Year 2001-2000 http://www.nrel.colostate.edu/IBOY/index2.html
USDA Plants Database http://plants.usda.gov/
Field Techniques
http://www.forestry.uga.edu/warnell/service/
http://forestry.about.com/science/forestry/library/weekly/aa121398.htm
Maps
http://terraserver.microsoft.com/
http://www.research.digital.com/SRC/personal/birrell/reliefMaps/
LAB EXERCISE: FOREST VEGETATION SAMPLING
This exercise demonstrates two different sampling procedures used to quantify the woody vegetation. The two sampling procedures do not measure the same things, so the results are not directly comparable.
METHODS
I. Angle Gauge Data Collection
1.Establish a 100m transect through the forest. Keep your transect at least 50m from other groups. If possible, have the transect run North-South or East-West. Note compass heading, slope aspect, topography, and general community characteristics (i.e. open stand of ponderosa pine, signs of previous fire or logging, etc.). Your sampling points will be every 10m, That is, from the base point of the transect, move ahead 10m and take the first sample. Thus, you will have data for 10 sampling points.
2. Stand at each sample point along the transect and survey the surrounding trees with the angle gauge. Hold one end of the gauge below your eye with one hand and the BAF gauge (the upturned end of the angle gauge) in the other hand with your arm extended.
3. Turn 360o and inspect each tree in view. Count and identify each tree that is as wide as or wider than the BAF gauge. Record the identity of each tree sampled. Use abbreviations for spp. as given to you by the instructor.
This lab generates a lot of numbers so it is important to properly tabulate your data. It may be more time efficient to have one person tabulate the data while others collect it. EVERYONE should take a turn collecting and recording data. The following table format will keep all of the information in order:
|
POINT |
sp.1 |
sp. 2 |
sp.3 |
spp. as encountered . . . |
|
1 |
7 |
9 |
1 |
. . . |
|
2 |
8 |
2 |
1 |
. . . |
|
3 |
(etc.) |
|
|
|
II. Point Centered Quarters Data Collection
1. Use the same transect and sampling points as established for angle gauge data collection. At each sampling point, stand facing the direction of the transect ahead of you. Try to get a mental picture of the four quadrants (quarter circles) around you.
2. Measure the distance from the sample point to the center of the nearest tree (dbh > 2 cm) in each quarter circle. Record the species identity, point-to-center distance in meters (measure to the nearest centimeter) and the dbh of the tree.
3. This exercise also generates a lot of data. The following format should be used for keeping it organized.
Point 1/4 1/4 1/4 1/4
1 sp.1 4.7 sp.2 2.0 sp.3 5.1 sp3 1.5
DBH 2.0 1.9 2.4 2.6
(etc.)
DATA ANALYSIS
To make sense of the data, we need to crunch it into some meaningful form. Likewise, statistical tests are necessary to determine whether or not values calculated among forests are significantly different from one another.
I. Angle Gauge
1. BASAL AREA (ft2/acre) BA =
(total # trees of a species/total # points) x 10
2. TOTAL BASAL AREA TBA = Sum of all basal areas
3. RELATIVE BASAL AREA FOR A SPECIES RBA = (BA/TBA) x 100
4. ABSOLUTE FREQUENCY AF = # points spp. measured at least once
5. INDIVIDUAL FREQUENCY IF = (AF /total # sample pts.) x 100
6. RELATIVE FREQUENCY RF = (IF/ sum of all IF) x 100
7. IMPORTANCE VALUE IV = RBA + RF
II. PCQ
A. For all spp.
1. MEAN DISTANCE D = (sum of distances for all spp. m /# pts. x 4)
2. MEAN AREA D2
3. TOTAL DENSITY PER HECTARE (/ha) T = 10,000/D2
B. For individual sp.
1. ABSOLUTE FREQUENCY AF = # number of points where a spp. was measured at least once
2. ABSOLUTE DENSITY PER HECTARE (/ha) AD =
(# quarters where a sp. was measured/total # quarters) x T
3. BASAL AREA (/ha) BA = (mean basal area per sp.) x AD {see table}
4. RELATIVE DENSITY RD = (# trees of a sp./# trees all spp.) x 100
5. RELATIVE FREQUENCY RF = AF / (sum of all spp. AF) x 100
6. RELATIVE DOMINANCE RB = BA / (sum BA all spp.) x 100
7. IMPORTANCE VALUE = RD + RB + RF
LAB EXERCISE: SAMPLING THE UNDERSTORY
Communities vary in species diversity and species richness. In describing the understory, or any community for that matter) we are often interested in the number of species present (richness). Richness alone is not a satisfactory measure of diversity, however. For example, a community consisting of 97 oak trees, 1 maple, 1 willow and 1 pine would have the same richness as a community composed of 25 members of each species although most people would recognize the former community as being less diverse. Thus, a number of indices have been developed which allow community analysis of not only the number of species present, but also their evenness. In addition to what species are present, we are also interested in how much space they occupy and how often they are found (ground cover). Below, a variety of techniques are described to sample and measure the understory.
I. Measures of Diversity and Richness
Whittaker or Plot-Frame method
1) Lay a 50 m transect through the community.
2) Choose an appropriate method for determining sample points along the transect.
3) Place a sampling frame (or make your own with a measuring tape) at you sampling point, search a 1 cm2 quadrat within the frame and note the number of species within it. The instructor will assist in determining how accurately species identification needs to be as well as use of appropriate keys.
4) Leaving the frame in the same location, search a 5 cm2 area for the presence of spp. not noted in the smaller quadrats.
5) Repeat step 4 in a single 1 X 1 m plot and in a single 20 X 50 m plot.
6) Plot cumulative number of species against increasing area and calculate a best fit linear regression for the line. The slope of this line can be compared among communities.
Simpson's and the Shannon Indices
1) Establish a 100 m transect.
2) Place a 1 m2 grid (or make a 1 m2 quadrat) at 20 m intervals along the transect. Record the species and numbers of individuals of each species found in each quadrat.
3) Calculate the following values for each quadrat where: No = total number of spp. in the sample; Xo = total number of individuals; Xi = number of individuals in sp. i; pi = Xi/Xo
Spp./unit area = No/ m2
Simpsons's index (C) = S (pi)2
Shannon index (H') = -S (pi)(lnpi)
4) Average spp./area, C, and H' across the 5 quadrats to obtain an overall value for a given community.
NOTE: Simpson's index is more sensitive to more abundant species and therefore weighs abundant species more than rare species. The advantage to this is that index values are unlikely to vary much from sample to sample because it is the rare species that will vary from place to place more so than the common species. The Shannon index is more sensitive to rare species. This index is thought to represent the "uncertainty" or "information" of a community. The more variable its composition, the more variable (uncertain or unpredictable) each sample of it would be. H' varies from 0 in a community of one species, to values over 7 in more diverse communities.
II. Measures of Ground Cover
Point Sampling
Ground cover by vegetation less than 1m can be easily measured with a point frame. The frame is placed on the ground with points arranged on the frame by means of pairs of crosshairs. Each pair of crosshairs can be lined up to accurately measure the vegetation or other ground cover found at a sampling point. Thus, this method provides a very unbiased means of measuring ground cover.
1) Lay a transect through a study area, and determine sampling intervals (regular or random) along the transect. At each point, flip a coin to decide which side of the transect to sample.
2) Standing above the frame, determine whether the crosshairs line-up with a plant, rock, bare ground, litter, etc. You may identify individual plant species or lump plants into broader categories depending upon your botanical expertise at species identification.
3) Calculate ground cover for different categories based upon the number of crosshairs intercepted by a type of ground cover divided by the number of crosshairs in a sampling point or in a transect. EXAMPLE: At a sampling point in your transect, rock was found to intercept 6 of the 10 crosshairs. Thus, at that point, rock comprises 60% of the ground cover. Likewise, if you measured 100 crosshairs along a transect, and grasses were found at 23 crosshairs, then grasses would comprise 23% of the ground cover along that transect.
Line-intercept Sampling
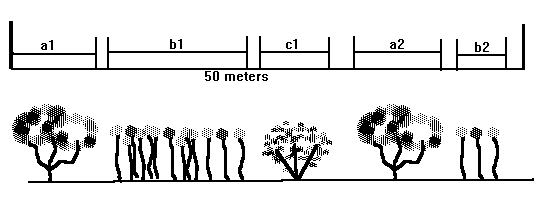
This technique is useful to measure shrub or large plant density along a randomly placed transect.
1) Lay a transect through the study area and measure the distances intercepted by individual plants of different species. (See figure below.)
2) Calculate percent cover by dividing the total distances intercepted by each species by the total of the transect length. You can also sum the distances intercepted over all transects and divide by the total lengths of all transects.

III. Determining Sampling Intervals
Before you begin sampling, you will want to determine whether you want to sample random or regular points along the transect. For regular sampling, simply determine a suitable distance along your transect for your needs. To sample at random, have one person hold a stopwatch (preferably with a digital seconds read-out) and start the timer. Have another person say, "Stop." Using the last digit in the seconds, move that distance (meters, steps, etc.) along the transect. Sample to the left of the transect if the number is odd or to the right if the number is even. Many other techniques for sampling can be used such as using random number tables, etc.