Figures 1 & 2
DAVID T. WEBB
Department of Biology, University of Puerto Rico, Rio Piedras, Puerto Rico C093 1 U.S.A.
Received February 2, 1982 Accepted March 9, 1982
Seedlings of Dioon edule
Lindl. cultured aseptically on agar slants of modified White's medium at pH 5.7 failed to develop root nodules in light or darkness. Light inhibited primary-root elongation and secondary-root production and stimulated callus formation from a periderm-like tissue which developed in both primary and secondary roots. Dioon edule, callus formation, periderm development, root growth inhibition, root nodulation.All cycads produce root nodules which may become inhabited by soil Cyanobacteria (Chamberlain, 1935). Members of the Zamiaceae from Australia (Lamont and Ryan, 1977; Webb, 1982 a) and North America (Webb, 1981; 1982 b) have been found to form root nodules in sterile culture. With Macrozamia riedlei nodulation occurred in darkness (Lamont and Ryan, 1977). However, with Bowenia serrulata (Webb, 1982 a) and Zamia pumila (Webb, 1981, 1982 b) light was required for nodulation. In these latter studies, light also inhibited primary-root elongation and secondary-root production. This study was designed to determine whether Dioon edule Lindl. which is a Mexican species in the Zamiaceae, formed root nodules in sterile culture and whether light inhibited primary-root elongation and secondary-root production by seedlings of this species.
Seeds of Dioon edule Lindl. were obtained from J. L. Hudson, Seedsman, Redwood City, California) and from Guerra's Inc., Mission, Texas. Seeds were prepared and cultured aseptically as previously described (Webb, 1981, 1982 b).
After 14 days of growth in the dark at 27 C, uniform seedlings were selected for further study. These were cultured in darkness or exposed to either 12 or 24 h photoperiods of fluorescent light (General Electric Cool White) at 27 C. Light intensity was measured at the level of the culture tubes with a Gossens Luna-Pro photographic light meter and with an International Light IL-SOC radiometer.
Each treatment contained six individuals. Statistical significance of mean growth measurements was tested at the 5% level with Wilcoxin's signed ranks test which has virtually the same sensitivity as the student's t test (Langley, 1970). Horizontal lines below the x axis indicate significance as determined by the signed ranks test. Treatments without overlapping lines are significantly different. Where results were obviously different, statistical tests were not used.
The anatomy of light and dark-grown primary and secondary roots was examined by making transverse free-hand sections and staining them with 0.005% aqueous Toluidine blue (Rock, 1981) or with 0.5% Sudan IV in 800/0 ethanol (Foster, 1949).
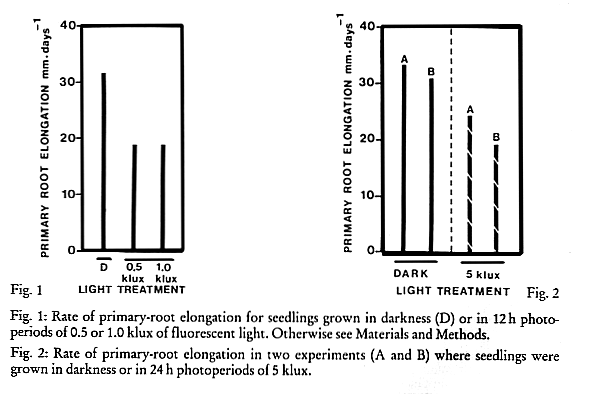
In one experiment where seedlings were exposed to 12 h photoperiods of 0.5 or 1 klux or to darkness, light at both intensities inhibited primary-root elongation by 40% (Fig. 1). In two experiments where seedlings were exposed to 24 h photoperiods of 5 klux or to darkness, light inhibited primary-root elongation by 27 and 38 % (Fig. 2). In these latter two experiments light severely inhibited secondary-root formation (Fig. 3).
Fig. 1: Rate of primary-root elongation for seedlings grown in darkness (D) or in 12 h photo-periods of 0.5 or 1.0 klux of fluorescent light. Otherwise see Materials and Methods.
Fig. 2: Rate of primary-root elongation in two experiments (A and B) where seedlings were grown in darkness or in 24 h photoperiods of 5 klux.
When these cultures were transferred to darkness secondary-root production commenced and continued at a rate comparable to that of the dark controls. However, only 10 and 12% of these latter formed roots developed from primary-root areas which had previously been exposed to light.
Callus formation occurred from 66% of the seedlings exposed to 24 h photoperiods of 5 klux (Fig. 4). Only 16% of the dark controls developed similar calluses and these were far less extensive than those formed in the light.
Callus formation also occurred at the junction of the primary-root and the shoot in all of the above cultures. When seedlings with light-induced calluses were transferred to the dark, the calluses retained their unorganized appearance but in a few cases secondary-roots grew out of these calluses.
Secondary-roots formed in all of the light treatments developed chloroplasts but otherwise resembled those produced by the dark controls (Fig. 5). When dark controls were transferred to 24 h photoperiods of 5 klux, callus formation occurred along the primary- and secondary-root axes (Figs. 6, 7) and from secondary-root apices (Fig. 7) in 83 % of the cultures. These latter calluses eventually became organized into typical root apices which did not resemble nodules (Fig. 6). Callus formed along the root axes remained unorganized.
In both primary- and secondary-roots callus formation also occurred in both light and darkness, where lateral roots emerged (Figs. 6, 7). These calluses were more prominent in the light than in the dark.
Callus formation not associated with lateral root emergence, had a superficial origin in the subepidermal cortex. The phellogen of developing periderm was continuous with areas of callus proliferation which resembled lenticils. Callus cells were isodiametric and were loosely arranged in uniseriate rows. The callus producing meristem appeared to be a single cell layer external to cortical parenchyma cells. Callus cells stained positively for carbohydrates with Toluidine blue but they failed to stain positively for suberin with Sudan IV. Adjacent phellem cells stained positively with Sudan IV.
Fig. 4: Primary-root (P) of seedling exposed to 24 h photoperiods of 5 klux for 53 days. Note the numerous areas of callus (C) formation along the
root axis. Dark areas are due to presence of periderm. Otherwise see Materials and Methods. 3:1.
Fig. 5: Secondary-roots (S) of dark control seedling after 53 days. Otherwise see Fig. 4. (3:1).
Fig. 6: Secondary-root of dark control after transfer to 24 h photoperiods of 5 klux for 50 days. Note callus (C) formation from primary (P) and secondary-root and presence of wound callus (WC)
where the secondary-root emerged from the
primary-root. Callus formed at the former apex (FA) has given rise to a typical root apex
(RA). 4:1.
Fig. 7: Same as Fig. 5 following 30 days exposure to 24 h photoperiods of 5 klux. Note callus formation at root apices (A) and at the sites of tertiary root (T) emergence. 4:1.
Light-induced callus which formed at different loci frequently united and completely encircled the root. Internally the callus originated from a single meristematic cell layer which was continuous around the root cortex. Following secondary growth of the vascular cambium, the callus producing meristematic layer was located in the secondary phloem. Cells produced by this lateral meristem were similar to those produced at the onset of callus formation and appeared to be the product of periclinal divisions in one direction. In no cases were lateral-root primordia or areas resembling them seen in association with these calluses or their meristems.
The development of callus formed by secondary-root apices was not examined.
The inhibition of primary-root elongation and secondary-root production by light observed in these experiments was similar to that observed with Z. pumila (Webb, 1982 b). However, with D. edule, inhibition of primary-root elongation was not related to the applied light intensity and was almost the same with 12 h photoperiods of 0.5 or 1 klux and 24 h photoperiods of 5 klux. With Z. pumila greater inhibition occurred with increasing light intensities, and 12 h of 5 klux was more inhibitory to primary-root elongation with Z, pumila than was 24 h of 5 klux with D. edule. With D. edule secondary-root production was more severely inhibited by 24 h of 5 klux than it was by 12 h of 5 klux with Z. pumila (Webb, 1982 b). In experiments with D. edule different seed lots and photoperiods were used in the two sets of experiments and this makes direct comparisons within these experiments and to experiments with 7. pumila tentative.
Secondary-roots of D. edule which developed in the light had a typical lateral-root morphology and were not nodular. Light exposure caused a dramatic increase in callus formation from primary- and secondary-roots. The origin and development of this callus conformed to that of periderm (Esau, 1965) but had accelerated growth and atypical differentiation. Esau (1965) reported that Kuster (1925) observed the formation of unsuberized callus from periderm exposed to excess moisture. Increased local moisture also led to accelerated periderm activity with Eucalyptus stems (Liphschitz and Waisel, 1970). With cultured roots of Ophioglossum application of benzyladenine and 2,4dichlorophenoxyacetic acid led to the formation of a periderm-like zone in the cortex of these roots which do not normally develop periderm (Peterson, 1971).
Light did not appear to play a significant role in periderm formation by Eucalyptus stems (Lipschitz and Waisel, 1970).
With Bowenia serrulata (Webb, 1982 a) and 7. pumila (Webb, 1981, 1982 b) light induced secondary-root nodulation. Since auxin (Slankis, 1950, 1958) and cytokinin (Becking, 1975) can induce root nodulation in other gymnosperms or in angiosperms and since these two hormones may be involved in periderm development (Peterson, 1971), it is logical to hypothesize that light alters the hormonal balance in cycad roots which leads to nodulation with B. serrulata and 7. pumila, and to periderm-derived callus formation with D. edule. Callus formation by D. edule roots may be enhanced by the moist culture environment.
The observation that nodulation did not occur with D. edule in sterile culture suggests that other abiotic and/or biotic factor(s) must be required for nodule formation. Within the Zamiaceae there appear to be three patterns of nodulation. With Macrozamia rudely nodulation does not require a specific external stimulus (Lamont and Ryan, 1977). With B. serrulata (Webb, 1982 a) and Z. pumila (Webb, 1981,1982 b) light causes nodulation and with D. edule (an) unknown external factor(s) is required for nodulation.
Dioon edule seeds necessary for these experiments were donated by Mr. William Steen of The Cycad Society. This research was supported by a grant from the Office of Graduate Education and Investigation, University of Puerto Rico, Rio Piedras.