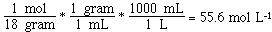Diffusion and Osmosis
First about Water!
Water is 90-95% of plant weight
All biochemistry in plant in an aqueous environment
Water is excellent polar solvent
High specific heat...heat buffer
Highest heat of vaporization...evaporative cooling
Cohesive...able to support tensions (-30 MPa) = 10% copper wire
Adhesion to surfaces (wetting)...surface tension
Capillarity (cohesion + adhesion) avoids cavitation.
Diffusion
movement of a substance from an area of high concentration to an area of lower concentration.
Fick's Law:

If distance is small (50 um) diffusion is about 2.5s.
If distance is large (1 m long corn leaf) diffusion is too slow = 24 years!
Does this apply to water movement? Not so easy!
What is the molarity of pure water?
density = 1 gram ml-1
molecular weight = 18 gram mol-1
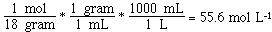
What is the molarity of sea water?
density = 1.025 gram ml-1 = 1025 gram L-1
concentration NaCl = 29.5 gram L-1
concentration water = 1025 - 29.5 = 995.5 gram L-1

Not such an impressive difference (55.6 vs 55.3)!
Movement of water must be more than diffusion based on concentration of water!
Longer distance movement of materials must rely on a faster process to be practical.
Bulk flow
movement of substance under influence of pressure
Poiseuille Equation:

This will work in xylem: note independence of solute concentration!
Evolution may work to increase vessel diameter (radius)4
Osmosis
passive movement of water across (through) selectively permeable membrane
This process is spontaneous, so it must be the result of a downhill energy system. We call this WATER POTENTIAL. Water spontaneously moves from an area of higher water potential to an area of lower water potential.

&psi = Water potential (the sum of factors) determines how water moves in a system. Units are MPa (or others).
&psis = Solute potential = the effect of dissolved substances in the water.
Distilled water = 0 MPa
1 M Sucrose @ 20 C = -2.436 MPa
Van't Hoff Equation: &psis = - CiRT
C= molar concentration
i=ionization constant
R=constant
T=absolute temperature
| RT = | 2.271 @ 0 C
2.436 @ 20 C
2.478 @ 25 C | L MPa mol-1 (table 3.2 p 69)
|
&psip = Pressure potential = the effect of hydrostatic pressure in the system.
0 MPa for STP (absolute 1 atm = 0.1 MPa)
tension/vacuum <0
turgor pressure > 0
&psig = Gravitational potential = the effect of height above sea level.
0 at sea level
0.1 MPa at 10 meters above that
&spig = density * gravity * height
density * gravity = 0.01MPa m-1
NOTE: interaction of pressure and gravity in fig 3.9 pg 69
&psim = Matric potential = the effect of colloids (adhesion) in soil or CW.
described on pg 76
Because matric potential is limited in cells, and typically we are looking at a tiny cell the height parameter is nearly = 0, the water potential expression simplifies to:
&psi = &psis + &psip
Take an example through a calculation or two:
A cell is about 0.3 M, what is its solute potential at 20C ? = -CiRT
- 0.3 * 2.436 = -0.731 MPa
Now we will assume the cell is full but has no turgor pressure initially.
Cell =
&psis = -0.731 MPa
&psip = 0 MPa |
| &psi = -0.731 MPa |
Now put this cell into a large volume of pure water at STP:
Water moves from area of higher potential (0 MPa) to area of lower potential (-0.731 MPa)
Assume rigid walls (reasonable but not perfect: see pg 70) so no volume or concentration change inside cell.
At equilibrium (no net movement of water) water potentials are equal:
&psis = 0 MPa
&psip = 0 MPa | | &psi = 0 MPa |
|
&psis = -0.731 MPa
&psip = 0.731 MPa | | &psi = 0 MPa |
|
The cell was placed in a hypotonic solution, gained water but mostly pressure increased (Got Crispy).
Now put the cell into a large volume of 0.1 M sucrose
&psis = - 0.1 * 2.436 = -0.244 MPa
At equilibrium water potentials are equal. Assume for now that sucrose cannot go into or out of the cell.
&psis = -0.244 MPa
&psip = 0 MPa | | &psi = -0.244 MPa |
|
&psis = -0.731 MPa
&psip = 0.487 MPa | | &psi = -0.244 MPa |
|
Now move the cell into 0.3 M Sucrose: &psis = - 0.3 * 2.435 = -0.731 MPa
&psis = -0.731 MPa
&psip = 0 MPa | | &psi = -0.731 MPa |
|
&psis = -0.731 MPa
&psip = 0 MPa | | &psi = -0.731 MPa |
|
The cell was placed in an isotonic solution, lost water but mostly pressure decreased (went limp)
Now move the cell into 0.6 M Sucrose &psis = - 0.6 * 2.435 = -1.461
Since the cell has no turgor and there is no internal structure to withstand tension (vacuum = negative potential), the water will continue to move out until the solute concentration is increased sufficiently to reach equilibrium:
&psis = -1.461 MPa
&psip = 0 MPa | | &psi = -1.461 MPa |
|
&psis = -1.461 MPa
&psip = 0 MPa | | &psi = -1.461 MPa |
|
How much cell volume is lost? About 50%, right (0.3 to 0.6 M)? The cell is plasmolyzed in this hypertonic solution.
How fast does the water move in osmosis or how fast do solutes move in diffusion?
FickÕs law tells us that: Js = -Ds&deltaC page 65
where:
Js = rate
Ds = Diffusion coefficient
&deltaC = concentration difference
another way to think of the diffusion coefficient is "permeability."
Some important ideas:
- Are membranes permeable?
- Are membranes semi-permeable?
- Are membranes selectively permeable?
- Are membranes differentially permeable?
In our worked example we ASSUMED that solutes cannot move in and out of cells. Is that realistic?
A phospholipid bilayer can be traversed only by very small compounds such as O2 CO2 and water. How DO cells acquire molecules of various sizes?
Diffusion-which solutes?
Facilitated Transport-which solutes?
Active Transport-which solutes?
Vesicular Transport (Endocytosis, Exocytosis, Phagocytosis, Pinocytosis)
How might we alter the permeability of a membrane?
Go back to the Course Schedule.