- Fundamental propterties of proteins
- 1¡, 2¡, 3¡, maybe 4¡ level of structure
- Heat labile
- Vesicular transport across membranes=huge
- Accelerate reactions by lowering activation energy
(NOT by altering amount of product made!)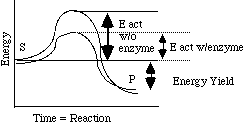
- Forms activated complex: E + S <-> ES <-> E + P
- Not used up ^ ^
- Effective at low concentration
- Specific to substrate used, reaction catalyzed, product made

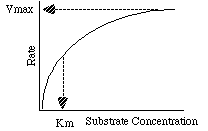
For this plot Km is the Substrate Concentration at 1/2 maximum rate.
The value of 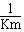 is the "affinity" of the enzyme for the substrate
is the "affinity" of the enzyme for the substrate
How do we get Vmax and Km?
- Inspection:
The eye-brain combination is a good integrator!
No statistical measures possible!
- Graphic method:
Do some algebra on Michaelis-Menten equation:

Now if we let:
 and
and  then similar to y = mx + b
then similar to y = mx + b
Which plots to a straight line with:
 and
and 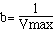
Lineweaver-Burke Plot:

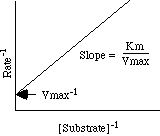
This plot lets us determine Vmax and Km with much less "subjectivity."
Vmax-1 = y intercept
-Km-1 = x interceptStatistical Method: linear regression of transformed data
- Statistical Method: nonlinear regression
This is the BEST way to do it as it uses the Michaelis Menten function directly to fit the curve to the data.