
Plant physiologists began to assist in the understanding of seed germination as the brewing industry sought to improve the alcohol yield in beer making. The industry already knew that it was important to sprout barley seeds. This process was accomplished by soaking seeds in water, then allowing the germination process to produce sugar from starch. The sugar produced is maltose (glu-glu, a disaccharide) so the sprouting process was called "malting." Getting more of this maltose produced was clearly the way to improve alcohol yield per bushel of barley seed.
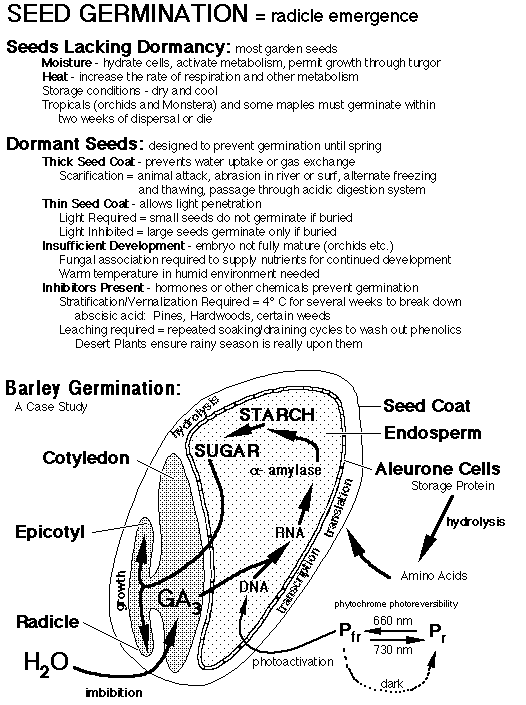
The diagram (far below) shows the basic process discovered by plant physiologists. You will notice that gibberellic acid (GA) is produced in the embryo. The imbibition process carries the gibberellic acid from the embryo to the endosperm. The aleurone cells (protein storage cells) toward the outside of the endosperm respond to this gibberellic acid. Because GA is made in one cell, transported, and another cell responds to it, GA has been named one of the several classes of plant hormones.

The GA stimulates the transcription and translation of alpha-amylase in the aleurone cells. The storage proteins serve as a source of amino acids for the translation process. The finished amylase is exported to the cells toward the interior of the endosperm. This is an exocytosis (secretion) process. There, the amylase catalyzes the hydrolysis of starch into maltose.
By treating the barley seeds with a solution of gibberellic acid (instead of plain water) the higher level of hormone signal causes more synthesis of amylase. This results in a large acceleration of maltose release.
In related research, plant physiologists wondered why people have trouble with seed germination of lettuce in their vegetable gardens. They knew that seed germination was very high in Petri dishes in the laboratory.
It turns out that in lettuce seeds, unlike barley, a critical step in triggering seed germination is photoactivation. The seeds need to be exposed to light in order to germinate. I hope you are wondering, immediately, how germination responds to photon flux and wavelength of light!
Lettuce responds well to very low photon fluxes...it is not a photosynthetic process!
The wavelength of light is critical. The seeds germinate well in white light, but also to single "colors"...particularly red light (660 nm). On the other hand, far-red light (730 nm) strongly reduces lettuce germination.
It took a long time to identify, isolate, and characterize the photoreceptor. It is called phytochrome. Phytochrome exists in two different chemical forms: Pr and Pfr. Phytochrome in its Pr form absorbs light maximally in red wavelengths...hence Pr. Phytochrome in its Pfr form absorbs light maximally in far-red wavelengths...hence Pfr. The name of the form of phytochrome is determined by the color of light it absorbs maximally. What made characterizing phytochrome difficult was the fact that the two forms interconvert. As Pfr absorbs far-red light, it changes chemically into Pr! Similarly, Pr absorbs red light and changes chemically into Pfr.
If you think about how you might analyze a pigment (as you have done several times in lab!), you generally put an extract into a spectrophotometer and measure absorbance of a wavelength at which the pigment maximally absorbs light. With phytochrome, this is almost impossible...the light you would use to measure it, causes it to change to the other form! It is elusive!
Back to the lettuce, obviously the garden problem is planting the seeds too deep (in the dark!). Without light to photoactivate seed germination, the seeds fail to germinate to their potential. In the dark, the ratio of Pfr to Pr determines whether each seed will germinate. If exposed to red light, the phytochrome is all converted into Pfr and the seeds germinate. When the seeds are exposed to far-red light, the phytochrome is all converted into Pr and the seeds fail to germinate. Obviously the active form of phytochrome is Pfr.
Go back to the Course Schedule.