Models for cytochrome c biogenesis
Three systems have evolved for the synthesis of cytochromes c.
Working models of the three distinct systems for cytochrome c biogenesis. Listed below the models are the organisms which utilize each system. All proteins shown in the cytochrome c compartments function specifically for the synthesis of c-type cytochromes, except the DsbA and DipZ proteins (system I). These latter proteins are general disulfide oxidizing and reducing enzymes, respectively.
Model for System I
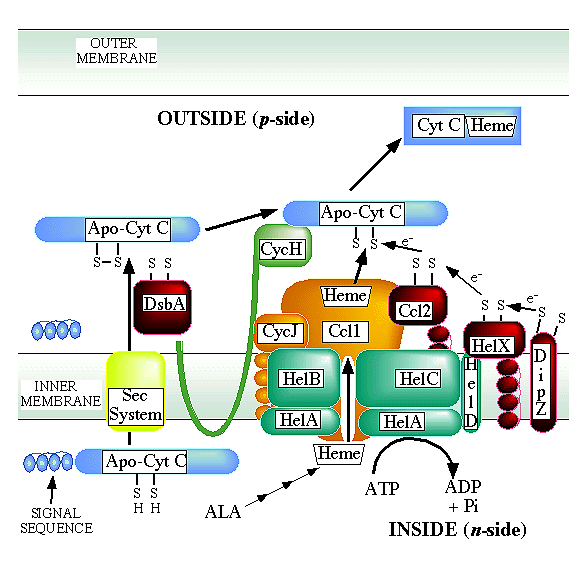
The system I model is built from bacterial studies.
This system is found in alpha and gamma proteobacteria, plant mitochondria, jakoboid flagellate mitochondria, Deinococcus and some Archae. This pathway can be thought of as two separate pathways: i) one to reduce the two cysteines of the apocytochromec--such thioredox proteins are colored RED (for simplicity, sulfhydryl groups (-SH) are shown as -S and S-S represent oxidized disulfide bonds); ii) a second pathway to deliver heme in the reduced state (BLUE or ORANGE). It is proposed that heme and apocytochrome c are brought together for ligation in the reduced state. The DsbA, HelX, and Ccl2 proteins have a typical thioredox motif (CXXC).
For System I genes, some other nomenclature used is as follows:
- helABCD=ccmABCD=cycVWorf263cycX
- helX=ccmG=cycY=tlpB=dsbE
- ccl1=ccmF=cycK
- ccl2=ccmH (N-terminus)=cycL
- cycJ=ccmE
- cycH=ccmH (C-terminus)=ccmI
top
Model for System II
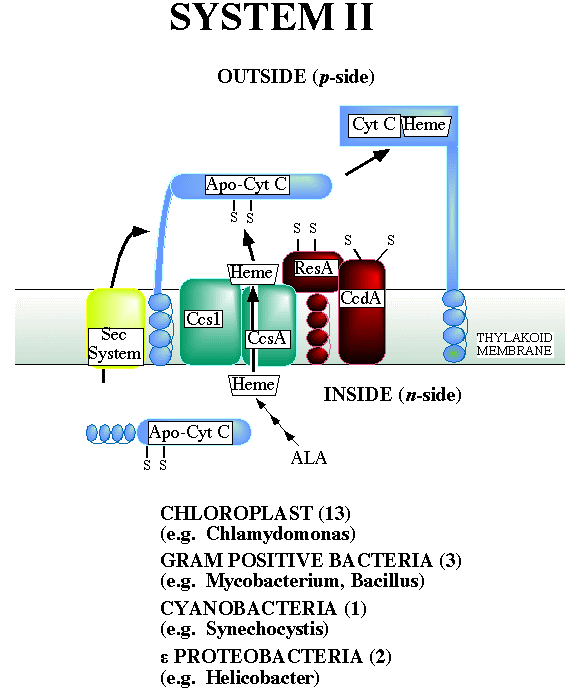
The model for system II is derived from genetic analysis of gram positive bacteria and Chlamydomonas.
System II is also found in the epsilon proteobacteria such as Helicobacter and the beta proteobacteria Neisseria. Reduction of the two cysteines of the apocytochrome c is predicted to be catalyzed by the ResA protein, which is rereduced by the CcdA protein. Both proteins contain a typical thioredox motif (CXXC). These proteins are colored RED. Heme is presumably delivered to the membrane surface in the reduced state by the CcsA and Ccs1 proteins (BLUE or ORANGE). It is proposed that heme and apocytochrome c, both in the reduced state, are brought together for ligation of the vinyl groups of heme to cysteinyl residues of the apocytochrome c.
top
Model for System III
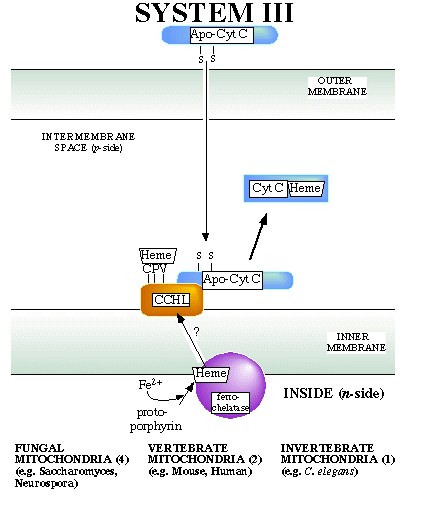
The system III model is derived from genetic and biochemical analysis of fungi such as Saccharomyces and Neurospora.
Cytochrome c heme lyase (CCHL) is involved in the transport of apocytochrome c from the cytoplasm by serving as a specific bonding site (trans side receptor). In addition, CCHL is essential for heme ligation.
top
To view a picture containing all three models
back to homepage
Those interested in obtaining high quality pictures of these models please contact Bob Kranz.



